What Is the Meaning of Thermodynamics for Engineers?
When discussing thermodynamics for engineers, one of the most widely cited definitions in technical literature is the following:
Thermodynamics definition: is the science that studies the transformations of energy and matter, and the relationships between the physical properties involved in these transformations.
(As stated in classic references such as Çengel and Boles, “Thermodynamics: An Engineering Approach”)
To fully understand this definition, it helps to see how thermodynamics integrates with the operative laws that govern real processes in physics, chemistry, biology, and engineering.
In each of these disciplines, transformations involving energy and matter occur regularly, taking place through observable, measurable, and often controllable phenomena.
This article starts from a fundamental assumption:
every real-world transformation—whether physical, chemical, biological, or technical—involves an exchange or variation of energy, matter, or both.
These changes produce effects that only thermodynamics can rigorously predict, interpret, and quantify.
In other words, thermodynamics tells us whether a transformation can occur, and under what conditions.
Operative laws, instead, explain how the transformation takes place: with what intensity, by which mechanisms, and over what timescale.
Only after establishing whether a process is thermodynamically feasible does it make sense to analyze how it can be carried out.
At that point, the specific equations of physics, chemistry, or engineering come into play.
To clarify this fundamental distinction, the following examples will illustrate how thermodynamics intersects with operative laws across major scientific and technical fields.
For a deeper dive, see the authoritative:
Thermodynamics: An Engineering Approach by Çengel and Boles — official publisher page:
https://www.mheducation.com/highered/product/Thermodynamics-An-Engineering-Approach-Cengel.html
Thermodynamics for Engineers and Operative Laws by Disciplines
In Physics
Heat conduction
When a solid body is heated, thermal energy flows from high to low temperature regions.
This transfer is described by Fourier’s law, which quantifies the rate of heat transfer.
Thermodynamics, however, answers a deeper question: will the system reach thermal equilibrium? Is entropy being generated?
Energy conversion
- A falling object converts gravitational potential energy into kinetic energy.
- Mechanics explains how this occurs, but thermodynamics ensures the conservation of energy through the First Law, and helps evaluate the irreversibility of the process due to friction.
In Chemistry
Spontaneity of reactions
A chemical reaction proceeds spontaneously only if the Gibbs free energy change (ΔG) is negative. Thermodynamics determines whether the reaction is feasible, while reaction kinetics governs how fast it occurs.
Phase equilibrium
- In distillation, liquid-vapor equilibrium is modeled using Raoult’s and Henry’s laws.
- Thermodynamics defines the conditions under which components separate, setting the theoretical limits of the process based on energy and entropy.
In Biology
ATP synthesis
- The production of ATP in mitochondria depends on electrochemical gradients.
- Thermodynamics explains why these gradients store usable energy and defines the efficiency boundaries of the process.
Active transport
- Cells actively transport ions across membranes against concentration gradients.
- This requires external energy, because thermodynamics states that spontaneous movement only occurs when energy decreases.
- Moving against this flow means work must be done.
In Engineering
Mechanical engineering – Heat engines
- A thermal engine converts heat into mechanical work.
- Thermodynamics, through the Second Law, establishes the maximum theoretical efficiency (Carnot cycle), while engineering models help design and optimize real machines.
Mechanical engineering – Refrigeration and heat pumps
- Thermodynamic principles define the Coefficient of Performance (COP) for refrigerators and heat pumps.
- Once the theoretical limit is known, operative laws help size compressors, valves, and heat exchangers.
Chemical engineering – Distillation columns
- Separation by distillation relies on phase equilibrium governed by Gibbs energy minimization.
- Thermodynamics tells us what is possible; mass balances and design methods like McCabe-Thiele determine how to achieve it.
Chemical engineering – Reactors
- Thermodynamic parameters such as heat of reaction and equilibrium constants define reaction feasibility.
- Kinetics and transport models determine how the reaction proceeds within an actual reactor.
Environmental engineering – Adsorption of pollutants
- The adsorption of contaminants onto solid surfaces is governed by thermodynamic feasibility (ΔG < 0).
- Isotherm models like Langmuir or Freundlich are only valid if the underlying transformation is thermodynamically allowed.
Environmental engineering – Solubility and partitioning
- Contaminants partition between phases such as water, air, and soil according to thermodynamic principles.
- Laws like Henry’s law explain distribution, while the feasibility and direction of the transfer remain defined by thermodynamics.
Three Real-World Thermodynamics for Engineers Cases
Boiling Water on a Stove
At standard atmospheric pressure (1 atm), thermodynamics tells us that water boils at 100 °C—this sets the physical threshold for the phase change. To reach and maintain this temperature, engineers use Fourier’s law of heat conduction to calculate the power required from the stove, taking into account heat losses and the thermal properties of the system.
Air Compressor – From 2 bar to 8 bar
In an ideal isentropic compression from 2 bar to 8 bar, the required work is about 145 kW, confirming the process is thermodynamically feasible. In practice, real-world inefficiencies reduce performance, and with an assumed efficiency of around 80%, the actual power needed rises to about 180 kW. Engineers use the polytropic equation and performance curves to size the motor and select appropriate equipment.
Ethanol–Water Distillation Column
Thermodynamics defines the azeotrope at 95.6% ethanol as the maximum purity achievable under atmospheric pressure, setting a theoretical limit for the separation. To approach this limit in a real distillation column, engineers apply MESH equations and design tools like the McCabe–Thiele method to determine the number of trays, the reflux ratio, and the column diameter.
Thermodynamics for Engineers principales:
After exploring how thermodynamics applies to real-world processes, it’s important to return to the theoretical foundation that governs all of these transformations. These are the core principles—known as the laws of thermodynamics—that define what is physically possible and what is not.
For engineers, understanding these laws is not just a matter of theory: it’s essential for evaluating feasibility, setting performance limits, and designing systems that respect natural constraints.
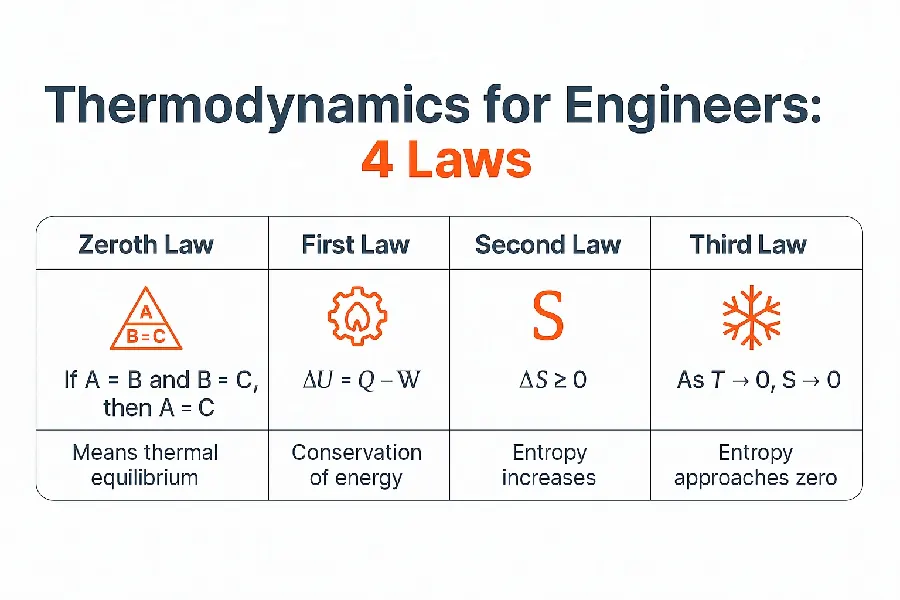
The Zeroth Law of Thermodynamics
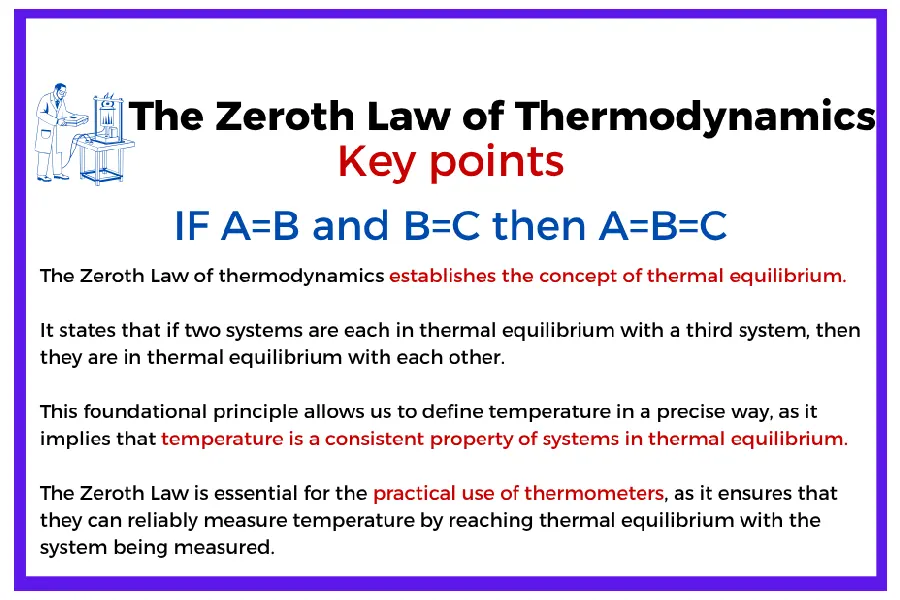
The Zeroth Law of Thermodynamics was formally stated only in the 1930s, after the first, second, and third laws had already been established and numbered.
However, scientists realized that this law was logically more fundamental—it actually came “before” all the others, as it defined the very concept of temperature, on which all subsequent thermodynamic formulations rely.
To avoid renumbering the already established laws, it was given the name “Zeroth Law,” a somewhat curious but now universally accepted solution.
It states that:
“If two systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other.”
This law, in its simplicity, forms the basis for temperature measurement: it allows us to compare systems thermally and justifies the existence of a consistent temperature scale. Thanks to this principle, we can say, for example, that two objects have the same temperature if no heat flows between them, and that a thermometer can serve as a reliable reference for determining the thermal state of a system.
Thermodynamics 1 law, thermodynamics energy balance equation
The first law of thermodynamics, often referred to as the law of energy conservation, states that energy cannot be created or destroyed—only transformed. This principle is expressed as:
ΔU = Q − W
Here, ΔU represents the change in internal energy of the system, Q is the heat added, and W is the work done by the system.
This law is applied in virtually every engineering context: compressors, turbines, heat exchangers, and closed chemical reactors all operate within its boundaries. It ensures that the total energy within a process is always accounted for.
For example, in the air compressor discussed earlier, the mechanical work input increases the internal energy of the gas, raising its pressure and temperature. The first law confirms that this energy must be conserved and accounted for.
In engineering applications, this concept is often extended into the thermodynamics energy balance equation, which considers not only internal energy, heat, and work, but also other forms of energy such as kinetic and potential energy.
For open systems or flowing fluids—such as turbines, compressors, or heat exchangers—the full energy balance is expressed as:
ΔH + Δ(½mv²) + Δ(mgz) = Q – W
Where:
- ΔH is the change in enthalpy (flow energy),
- Δ(½mv²) is the change in kinetic energy,
- Δ(mgz) is the change in potential energy,
- Q and W remain the heat added and work done.
This formulation is fundamental in engineering design because it allows engineers to account for all energy contributions, especially in non-static systems, ensuring accurate predictions and performance evaluations in real industrial processes.
The Second Law of Thermodynamics – Entropy and Irreversibility
Thermodynamics 2nd law, thermodynamics equilibrium
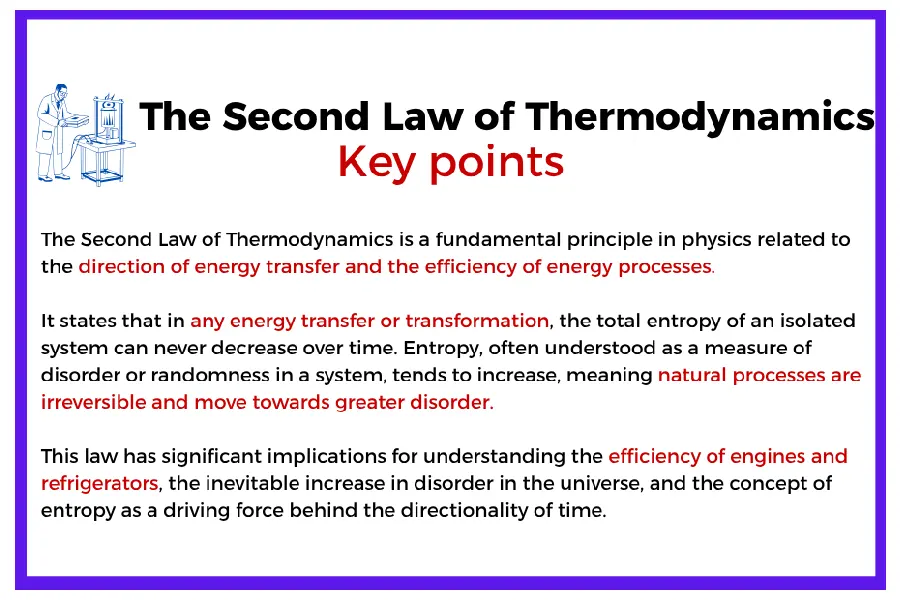
The second law introduces the concept of entropy and describes the direction in which processes naturally evolve. In an isolated system, total entropy can only increase or remain constant:
ΔS ≥ 0
This law explains why no machine or process is 100% efficient. It defines the limits of what can be achieved and highlights the irreversible nature of most real-world transformations.
A central concept related to the second law is thermodynamics equilibrium: the state in which a system’s macroscopic properties remain constant over time, and there is no net flow of energy or matter. At equilibrium, entropy is maximized under the given constraints, and no further spontaneous changes occur.
Consider the case of heat engines. Whether it’s a turbine, a piston engine, or a combined cycle, the second law sets a ceiling on efficiency—known as Carnot efficiency. No engine can surpass this theoretical maximum. Engineers rely on this principle to understand where real systems fall short and how to improve them within realistic boundaries.
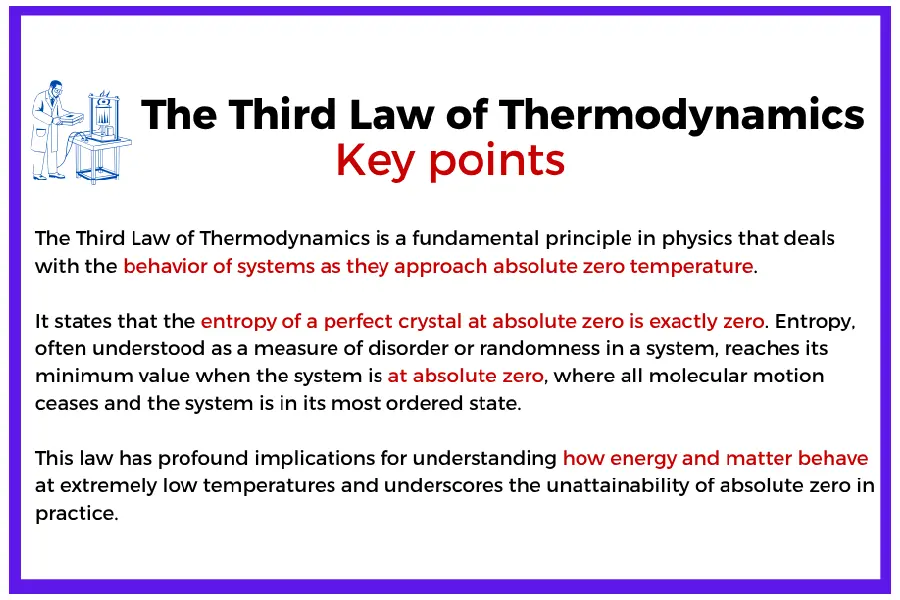
The Third Law of Thermodynamics – Absolute Zero and Entropy Behavior
(thermodynamics third law)

The third law of thermodynamics describes what happens to entropy as a system approaches absolute zero temperature. It states that the entropy of a perfect crystalline substance is exactly zero at absolute zero (0 K). In simplified terms:
As T → 0, S → 0
This principle sets a fundamental limit: absolute zero can never be reached in a finite number of steps. As temperature drops closer to 0 K, further reductions require disproportionately more energy and control.
In engineering practice, the third law becomes relevant in fields such as cryogenics, superconductivity, and ultra-low-temperature physics. For example, systems used to liquefy gases like helium, or to maintain superconducting magnets in MRI machines, must contend with increasingly steep thermodynamic and energetic barriers as they approach these extreme conditions.
This law not only deepens our understanding of entropy but also reinforces the physical boundaries within which real-world processes must operate—especially when dealing with extreme thermal conditions.
Thermodynamics Problems Engineers Actually Face (And How to Solve Them)
Studying thermodynamics is one thing. Applying it to real-world problems is another. Engineers regularly face thermodynamics problems that go far beyond textbook exercises. These challenges require a deep understanding of principles and the ability to connect formulas with physical intuition.
Let’s look at a practical example that combines the concepts of heat transfer, energy balance, and power usage.
Example: How Long Does It Take to Heat Water?
Imagine you’re designing a system to heat water in a storage tank. You know how much energy is required, and the heater has a fixed power output. A typical question could be: How long will it take to heat the water from 20°C to 80°C using a 5 kW heater?
Step 1: Calculate the heat needed (Q)
We use the standard formula:
Q = m × cₚ × ΔT
Where:
- m is the mass of the water (in kg)
- cₚ is the specific heat of water (4.18 kJ/kg·K)
- ΔT is the temperature difference (in °C or K)
Let’s assume we have 100 liters of water, which equals 100 kg (since water has a density of approximately 1 kg/L), and we want to raise the temperature by 60°C (from 20°C to 80°C):
Q = 100 kg × 4.18 kJ/kg·K × 60 K = 25,080 kJ
Now convert to joules (1 kJ = 1,000 J):
Q = 25,080,000 J
Step 2: Use the formula t = Q ÷ P
Now apply the time formula:
t = Q ÷ P
Where:
- Q is the energy required (in joules)
- P is the power of the heater (in watts)
P = 5,000 W
So:
t = 25,080,000 J ÷ 5,000 W = 5,016 seconds
Convert seconds to minutes:
t ≈ 83.6 minutes
What This Means for Engineers
In this example, the formula t = Q ÷ P—a rearranged form of the definition of power—is used to calculate how long a heater will take to deliver a specific amount of thermal energy. This is a typical engineering problem where thermodynamic principles meet practical constraints, like heater capacity, time limitations, and safety margins.
These types of problems occur daily in process industries, HVAC design, environmental systems, and power plants. What matters is not just knowing the equations but also interpreting the result:
- Is this heating time acceptable in an industrial setting?
- Should we increase the power or preheat the fluid?
- Is thermal insulation needed to reduce losses?
Thermodynamics gives you the tools to ask—and answer—those questions.
Example 2: Work Required to Compress Air in an Isentropic Process
Imagine you’re designing a compressor to raise the pressure of air from 1 bar to 8 bar. The air enters the compressor at 300 K, and the flow rate is 0.5 kg/s. The process is assumed to be isentropic — that is, reversible and adiabatic, meaning there is no heat exchange with the surroundings and no generation of entropy.
To find the ideal work required per kilogram of air, use the formula:
W = [n / (n – 1)] × R × T₁ × [ (P₂ / P₁)^((n – 1)/n) – 1]
Where:
R = 287 J/kg·K (gas constant for air)
n = 1.4 (isentropic exponent for air)
T₁ = 300 K (inlet temperature)
P₁ = 1 bar, P₂ = 8 bar (initial and final pressures)
Now plug in the values:
W = (1.4 / 0.4) × 287 × 300 × [ (8 / 1)^(0.4 / 1.4) – 1 ]
W ≈ 3.5 × 287 × 300 × (2.297 – 1)
W ≈ 3.5 × 287 × 300 × 1.297
W ≈ 307,860 J/kg
Now multiply by the mass flow rate to get the total power:
Ẇ = m × W = 0.5 × 307,860 = 153,930 W = 153.9 kW
The compressor must supply approximately 154 kW of power to achieve this pressure rise under ideal isentropic conditions.
In thermodynamics, an isentropic process is one in which the entropy of the system remains constant. This occurs when a process is both adiabatic (no heat is exchanged with the environment) and reversible (no internal dissipation or losses). While real processes are never perfectly isentropic, this assumption provides a useful benchmark for evaluating the minimum work required in systems like compressors, turbines, and nozzles.
Conclusion
Thermodynamics for engineers is often perceived as an abstract or purely theoretical discipline, but it is a powerful and essential tool. Understanding the fundamental laws of thermodynamics—energy conservation, entropy increase, and the behavior of systems near absolute zero—allows engineers to evaluate whether a process is possible, what its limits are, and how to approach it efficiently.
From heat exchangers to chemical reactors, from air compressors to distillation columns, every engineering decision involving energy or matter transformation is shaped by thermodynamic principles. As we’ve seen through examples, real-world problems often go beyond textbook cases and require a combination of theoretical insight and practical judgment.
Whether you’re a student approaching the subject for the first time or a professional looking to deepen your understanding, mastering thermodynamics means more than memorizing equations—it means learning to ask the right questions, interpret physical limits, and design solutions that work within the laws of nature.
Thermodynamics for engineers is not just about “knowing the laws.” It’s about using them to make better decisions, safer systems, and more sustainable technologies.
Ing. Ivet Miranda
Looking to Build a Stronger Foundation in Chemical Engineering? Start Here:
- What Is Distillation and How It Works in Real Industrial Processes
- Chemical Engineering Guide: 14 Useful Topics for Beginners
- Unit Operations: Useful 5 Core Units in Chemical Engineering
- The 4 Safety Management System Pillars Explained in Detail
- What Does a Chemical Engineer Do? 8+ valuable Roles
- 5 Key Differences: Rupture Disc vs Relief Safety Valve
FAQ
What is a thermodynamic system?
A thermodynamic system is a defined region in space where energy exchanges are studied—open, closed, or isolated.
What is thermodynamic equilibrium?
It’s the state in which no net flow of energy occurs within the system or between system and surroundings—mechanical, thermal, and chemical balances are all satisfied.
What is thermodynamics in physics?
It’s the branch of physics that studies heat, energy, and work, and how these quantities behave in different systems.
What is thermodynamics in biology?
Thermodynamics in biology explains how energy is produced, transferred, and used in cells—like in ATP production and metabolic pathways.
What is thermodynamics with example?
An everyday example: ice melting in a glass. Heat transfers from the environment to the ice, increasing entropy and causing a phase change.
What is a thermodynamic cycle?
It’s a series of processes in which a system returns to its initial state. Examples include the Carnot and Rankine cycles in engines.
What is thermodynamics used for?
It’s used in engineering, chemistry, biology, and environmental science to model energy efficiency, engine performance, reactions, and more.
What are the applications of thermodynamics?
From designing engines and refrigerators to understanding biochemical reactions and climate systems—thermodynamics applies broadly across science and industry.
What is the difference between enthalpy and internal energy?
Internal energy is the energy stored inside a system. Enthalpy includes internal energy plus flow work, making it useful for processes involving fluid flow.
What are the four laws of thermodynamics for engineers?
The four laws – Zeroth, First, Second and Third – define temperature equilibrium, energy conservation, entropy behaviour and the entropy limit at absolute zero. Together they set the theoretical boundaries for every engineering process.
See the full section above on the four laws for the equations.