What Are the Laws of Thermodynamics?
The laws of thermodynamics are a set of fundamental principles that govern how energy is transferred, transformed, and degraded in physical systems. They apply to all engineering processes involving heat, work, and energy interactions, regardless of scale or application.
Rather than describing specific devices or materials, these laws define universal constraints: what transformations are possible, in which direction processes naturally occur, and which performance limits can never be exceeded. For this reason, thermodynamics does not tell engineers how to build a system, but what no system can violate.
Together, the four laws of thermodynamics provide the conceptual framework used to analyze engines, power plants, chemical reactors, heat exchangers, and all energy-related industrial systems. Each law addresses a different aspect of energy behavior, from the definition of temperature to the limits of efficiency.
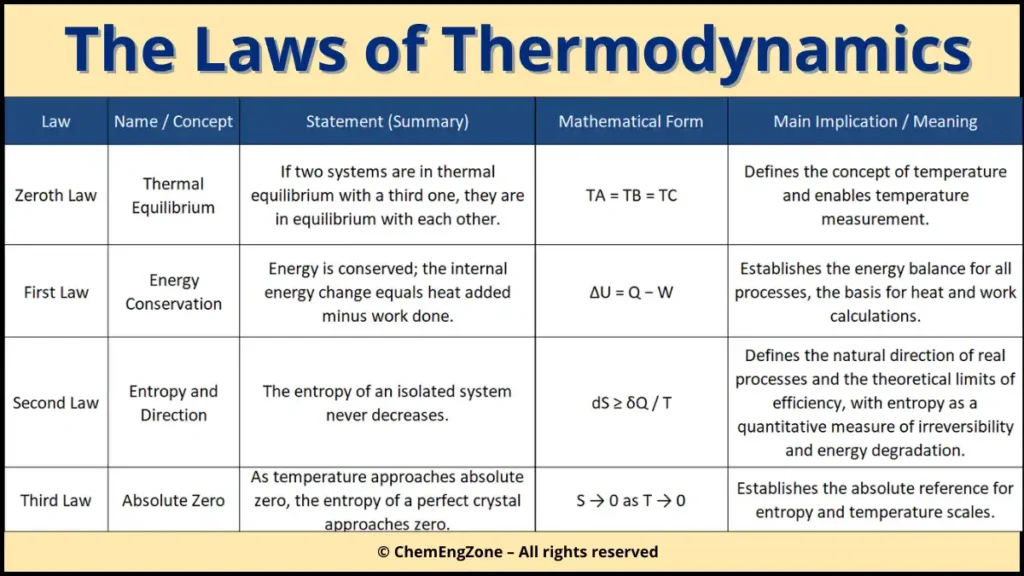
Zeroth Law of Thermodynamics: Thermal Equilibrium
The Zeroth Law of Thermodynamics establishes the concept of thermal equilibrium, which is the foundation of temperature measurement. It states that if two systems are each in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other.
Although introduced after the First and Second Laws, the Zeroth Law is conceptually prior to them. Without it, the concept of temperature would have no physical meaning, and comparisons between thermal states would not be possible.
This law explains why thermometers work. When a thermometer is placed in contact with a system and reaches thermal equilibrium, it shares the same temperature as the system. In this way, temperature becomes a measurable and transferable property, independent of the specific nature of the materials involved.
From an engineering perspective, the Zeroth Law provides the basis for defining temperature scales and for analyzing heat transfer processes between systems.
First Law of Thermodynamics: Energy Conservation
The First Law expresses the principle of conservation of energy.
Energy cannot be created or destroyed. It can only be transferred or transformed.
In thermodynamic form:
ΔU = Q − W
where internal energy changes due to heat exchange and work interactions.
The First Law introduces two fundamental ideas:
- Internal energy is a property of the system.
- Heat and work are modes of energy transfer, not properties.
However, the First Law says nothing about whether a process can occur spontaneously, nor does it impose limits on efficiency.
For a detailed experimental foundation of energy conservation, see the article on Joule’s Experiment & First Law of Thermodynamics.
For a complete treatment of mechanical work and PV diagrams, see Work in Thermodynamics. PV Diagrams.
Second Law of Thermodynamics: Direction and Irreversibility
The First Law guarantees energy conservation, but it does not explain why heat flows from hot to cold, why friction generates heat, or why no engine can reach 100% efficiency.
The Second Law introduces entropy and establishes the natural direction of processes.
In isolated systems:
Entropy can remain constant only in the ideal reversible limit.
In all real processes, total entropy increases.
This means:
- Heat cannot be completely converted into work in a cyclic process.
- Every real heat engine must reject part of the absorbed heat.
- Irreversibility is unavoidable.
The Second Law defines efficiency limits and excludes the possibility of a Perpetual Motion Machine of the Second Kind.
A full mathematical treatment, including the Clausius inequality and PMM2 analysis, is provided in Second Law of Thermodynamics: PM 2nd Kind.
Third Law of Thermodynamics: Absolute Entropy and the Limit of Cooling
The Third Law concerns the behavior of systems as temperature approaches absolute zero.
It states:
As T → 0 K, the entropy of a perfect crystalline substance approaches zero.
This law establishes an absolute reference for entropy values. Unlike energy, which can be arbitrarily defined relative to a reference level, entropy requires a defined zero point.
An important consequence is that absolute zero cannot be reached in a finite number of steps. No physical process can cool a system exactly to 0 K.
From an engineering perspective, the Third Law becomes relevant in:
- Cryogenic processes
- Low-temperature physics
- Absolute entropy calculations
It completes the thermodynamic framework by defining the lower boundary of temperature.
Logical Structure of the Four Laws
The laws form a progressive conceptual hierarchy:
Zeroth Law → Defines temperature and equilibrium
First Law → Defines energy conservation
Second Law → Defines direction and limits of processes
Third Law → Defines absolute entropy reference
Together, they describe:
- What can be measured
- What is conserved
- What direction processes follow
- What limits cannot be surpassed
Access the FREE PDF – Thermodynamics Overview
Download a two-page A3 visual summary covering the key concepts of thermodynamics: energy, work, heat transfer, and the fundamental thermodynamic laws. Designed to help you fix the concept quickly.
Continue to Access the PDFConclusion
The Laws of Thermodynamics are not independent rules, but interconnected principles that define the physical boundaries of engineering systems.
The Zeroth Law makes temperature meaningful.
The First Law ensures energy conservation.
The Second Law imposes irreversibility and efficiency limits.
The Third Law defines the absolute reference for entropy and the unattainability of absolute zero.
Every real industrial process operates within these constraints. No design, innovation, or optimization can bypass them.
Thermodynamics therefore does not describe how to violate nature — it describes the framework within which all engineering must operate.
Ing. Ivet Miranda
Thermodynamics Laws Quiz
Which thermodynamic law introduces irreversibility and sets limits on the efficiency of heat engines?
Other Articles You May Find Useful
First Law of Thermodynamics and Joule Experiment
Second Law of Thermodynamics: No Perpetual Motion
Chemical Engineering Principles Explained
Unit Operations: A Practical Introduction for Engineers
Heat Transfer Basics for Engineers
Heat Exchanger Fouling and Rubby Formation
Career Opportunities in Chemical Engineering
Vacuum Tank Collapse: Hazards & Prevention
FAQ
What is reversible work in thermodynamics?
Reversible work is the maximum possible work obtained from a process carried out infinitely slowly, with the system in equilibrium at every stage. It is represented by the exact area under the curve in the P–V diagram.
How many laws of thermodynamics are there?
Some modern discussions refer to additional principles, but the Zeroth, First, Second, and Third Laws are the universally accepted thermodynamic laws.
Is thermodynamics a theory?
Thermodynamics is not just a theory; it is a set of experimentally validated laws (Zeroth, First, Second, Third Law) that describe how energy is conserved and transformed.
Is thermodynamics physics or chemistry?
Thermodynamics is a fundamental branch of physics, but it is also applied in chemistry, engineering, and materials science. It studies energy transformations in all these contexts.