
What is Distillation Process?
Distillation is a method used to separate the components of a liquid mixture by taking advantage of the fact that they evaporate at different temperatures.
According to the first law of thermodynamics, when a substance is heated, it absorbs energy in the form of heat. This increases the kinetic energy of its molecules — in other words, they move more — until they begin to transition into the vapor phase.
The ease with which a substance evaporates is called its volatility.
Components with higher volatility evaporate first. During distillation, the mixture is heated and the vapors of the different components are collected separately, then cooled and condensed back into liquid form.
The entire process relies on the equilibrium between the liquid and vapor phases, which depends on the properties of the substances involved and the operating conditions.
These phase equilibria can be described quantitatively — in the case of ideal mixtures — using Raoult’s Law, which allows us to predict the composition of the vapor in equilibrium with the liquid phase.
Partial Vaporization Operations (Flash)
As shown in Figure 1, a mixture composed of C components, with initial composition x01,x02,…,x0C and flow rate L0, is subjected to an initial pressure P0 and preheated to a temperature T0.
The mixture then passes through an expansion valve, where it undergoes a sudden pressure drop down to a value P.
If the temperature is sufficiently high, the pressure drop results in two streams at the outlet of the flash drum:
– a liquid stream L with composition x₁, x₂, x₃, …, x𝒸.
– and a vapor stream V with composition y₁, y₂, y₃, …, y𝒸.
These two phases are in thermodynamic equilibrium, meaning that the vapor and liquid phases of each component are in equilibrium.
The flash vaporization process provides the fundamental insight needed to understand column distillation.
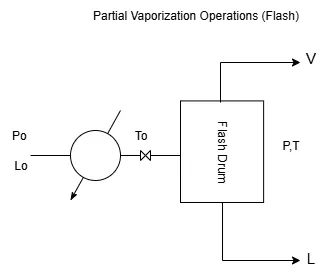
In a flash unit, a single vapor-liquid equilibrium is achieved after a pressure drop and partial vaporization.
A distillation column, by contrast, consists of a vertical sequence of equilibrium stages, where rising vapor and descending liquid repeatedly exchange mass and energy, driving the separation further at each level.
Distillation Column – Step-by-Step Explanation
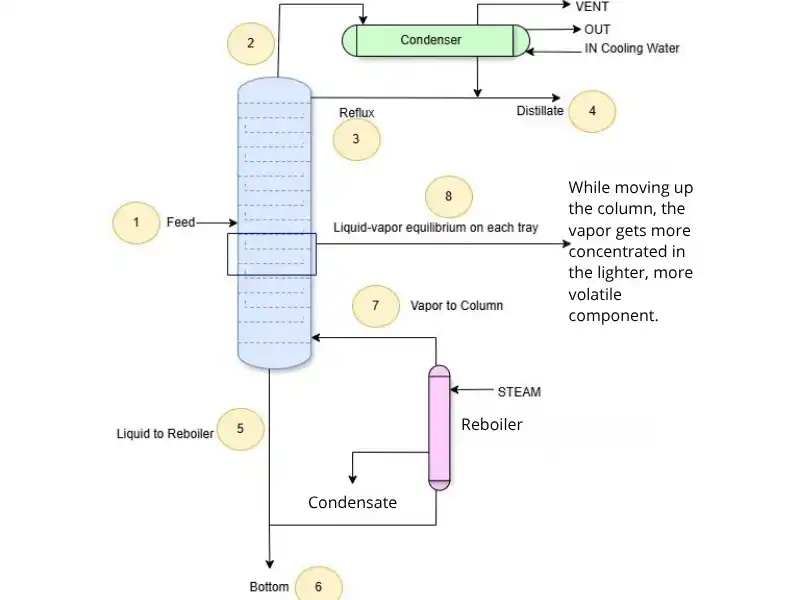
Unlike flash vaporization, where a pressure drop causes one-time separation, a distillation column achieves separation gradually, through repeated vapor-liquid contact across many equilibrium stages.
Step-by-step explanation using the diagram:
1. Feed (Entry Point)
A liquid mixture is introduced at a certain tray inside the column. This is the feed, and its position depends on the volatility of the components.
2. Vapor Rises – No Expansion Needed
Steam generated in the reboiler (bottom) produces vapor that rises through the column.
3. Liquid Falls
Condensed liquid from the top (reflux) and excess liquid from the feed flow downward by gravity. This creates countercurrent contact between vapor and liquid.
4. Equilibrium on Each Tray (or Packing)
At every tray, the rising vapor meets the falling liquid.
– They are not initially at equilibrium,
– But they exchange mass and energy until they approach local vapor-liquid equilibrium.
This is similar to a flash, but driven by composition difference, not pressure.
The separation is possible because the more volatile component prefers the vapor phase, and becomes increasingly concentrated as vapor rises.
5. Reboiler
At the bottom, part of the liquid is vaporized by adding heat. This generated vapor re-enters the column, continuing the separation.
6. Condenser & Reflux
At the top, the vapor is condensed. Part of it is collected as distillate, and part is returned as reflux, helping maintain efficiency and sharp separation at the top trays.
This example represents a continuous distillation column, a typical configuration used in industrial chemical processes.
The presence of both a reboiler (at the bottom) and a condenser (at the top) characterizes distillation as a complex unit operation in chemical engineering, involving simultaneous heat and mass transfer over multiple equilibrium stages.
Common Types of Distillation
n industrial practice, distillation is not a one-size-fits-all process. Several configurations exist to meet different needs, depending on factors such as feed composition, thermal sensitivity, desired purity, and energy efficiency. Here are some of the most common types of distillation used in chemical engineering:
Case 1 – Steam Injection Instead of Reboiler
In some distillation systems, especially when the bottom product is almost pure water and does not require further concentration, the reboiler can be replaced by direct steam injection into the bottom of the column.
This configuration is advantageous because it simplifies equipment design, reduces fouling at the bottom, and eliminates the need for a temperature gradient between the heating fluid and the liquid.
As a result, less energy is consumed, since the system no longer relies on an external heat exchanger operating with a temperature difference. The steam provides the necessary vapor directly, with minimal thermal losses.
Case 2 – Batch Distillation for Small Volumes or Variable Mixtures
In industrial and laboratory settings where the feed mixture is available in small quantities or its composition varies significantly from batch to batch, batch distillation is often preferred over continuous systems.
This approach is useful when flexibility is more important than throughput. Since the entire charge is loaded into the still at once, there is no need to reach steady-state operation, and the column can be adjusted easily for different feed compositions.
Batch distillation is especially suited for the production of high-purity solvents, fine chemicals, or when dealing with campaign processes in pharmaceutical or specialty chemical manufacturing.
The main advantage lies in its operational simplicity and adaptability. Continuous systems, by contrast, require stable feed conditions and longer startup times, which are inefficient for small or irregular production volumes.
Case 3 – Vacuum Distillation for Heat-Sensitive Mixtures
When the components in a mixture are sensitive to high temperatures, operating the distillation column under vacuum reduces their boiling points.
This prevents thermal degradation, which is especially important in the processing of pharmaceuticals, perfumes, and fine chemicals.
Lowering the pressure allows separation to occur at milder thermal conditions, making the process safer and more efficient for heat-labile substances.
Case 4 – Physically Separated Stripping and Rectifying Sections
In some processes, the rectifying (top) and stripping (bottom) sections of the column are optimized independently, either physically or by control strategy.
This is done when products require very precise specifications in purity or recovery.
By treating the two sections separately, engineers can fine-tune energy distribution, reflux ratio, and bottom reboiling rate to improve performance.
Case 5 – Azeotropic Distillation with Entrainers
In some binary mixtures, such as ethanol and water, the components form an azeotrope: a specific composition at which the vapor and liquid phases have identical compositions.
At this point, standard distillation is no longer effective, because the separation stops — the vapor produced has the same composition as the boiling liquid.
To overcome this limitation, an entrainer (a third component) is introduced to alter the vapor–liquid equilibrium.
This breaks or shifts the azeotropic point, allowing the components to be separated beyond their natural limit.
Azeotropic distillation is widely used when high-purity products are needed and azeotropes limit conventional separation techniques.
Case 6 – Steam Distillation for Immiscible or Heat-Sensitive Components
Steam distillation is used when the components to be separated are immiscible with water and/or decompose at high temperatures.
By injecting steam directly into the column, the total vapor pressure increases, allowing the components to boil at temperatures lower than their normal boiling points.
This method is particularly useful for extracting essential oils, aromatic compounds, or purifying natural products.
The advantage is that thermal degradation is avoided, since the effective boiling occurs at a lower temperature due to the combined pressure of steam and volatile compounds.
The possible configurations of distillation are numerous.
Each one presents its own characteristics, operating principles, and challenges, and deserves a deeper and case-specific study.
Distillation equipment
Tray columns and packed columns are two common types of distillation equipment, each with distinct characteristics.
Tray columns consist of horizontal plates stacked vertically, where vapor bubbles through the liquid in a stage-wise manner.
In contrast, packed columns are filled with structured or random packing material that provides a continuous surface for vapor–liquid contact.
Tray columns operate through discrete steps and tend to have a higher pressure drop, but offer predictable performance and are easier to clean and inspect.
They are typically used in large-diameter systems and are more tolerant to fouling or foaming liquids.
Packed columns allow continuous contact between vapor and liquid, with lower pressure drop and better efficiency under vacuum.
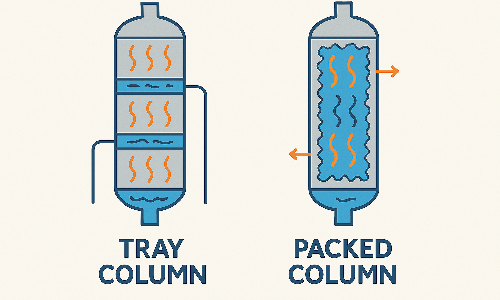
They are ideal for smaller-diameter columns and are commonly used in fine chemical processing or gas absorption.
While tray columns generally involve higher capital costs, packed columns offer a more economical solution when conditions permit.
From a maintenance point of view, tray columns are easier to inspect and clean because the trays are accessible.
This makes them more suitable for systems prone to fouling or scaling. Packed columns, especially those with random packing, are harder to clean and may require full replacement if clogging occurs.
You can learn more in our article: What is the process of distilling water, alcohol, and oils.
Thanks for reading!
Ing. Ivet Miranda
⬆️ Back to TopLooking to Build a Stronger Foundation in Chemical Engineering? Start Here:
FAQs
Why distillation is important?
Distillation matters because it gives us a reliable, scalable way to take a mixed liquid (or liquid + vapor) stream and pull out one or more components in a purer, usable form.
What are distillation fractions?
Distillation fractions are the different portions or cuts of a mixture that are separated during a distillation process based on their boiling points. Each fraction is collected over a specific temperature range and typically contains compounds with similar volatilities.
Distillation – How Does It Work?
Distillation works by separating the components of a liquid mixture based on differences in their boiling points. When you heat the mixture, the more volatile components vaporize first. These vapors are then condensed back into liquid and collected separately. This allows you to isolate one or more pure substances from a mixture. Learn more in the section:
How does distillation work?
Why is the distillation rate important?
The distillation rate—the speed at which vapor is condensed and collected—directly affects product quality, efficiency, and process control in both laboratory and industrial distillation. It’s not just about how fast you can collect a product, but how well the separation works.
What is the difference between tray and packed columns?
Tray columns use horizontal plates to separate the mixture step by step, while packed columns use special materials to create a continuous surface for contact. Tray columns are better for large flows and easier to clean; packed columns are more efficient for low-pressure operations and smaller systems.